Premature ovarian insufficiency (POI), also known as premature menopause, is the loss of ovarian function in women under 40 years of age. It has multiple adverse short- and long-term impacts on women’s psychological and physical health. Women may present with secondary oligo/amenorrhoea or infertility. However, the diagnosis is often delayed. The aim of this article is to present a typical case scenario and discuss the management of POI with a focus on infertility.
Case
Susan, aged 38 years, is an accountant who presents to her general practitioner (GP) to discuss her desire for a pregnancy. She had her first period at 13 years of age and has been on the combined oral contraceptive pill (COCP) ‘on and off’ for the past 20 years, including continuously for the past five years. During the times that she was not using the COCP, she has had regular periods. She has had no previous pregnancies.
Susan stopped the COCP five months ago and has not been using contraception with her long-term male partner. Before ceasing the COCP, she had a routine pre-pregnancy check-up with her GP. After stopping the COCP she had some spotting initially, and two days of spotting a few weeks later, but no other bleeding. She had no vasomotor symptoms but feels tired. Her friends told her that it might take a few months for her periods to return after being on the COCP for an extended time. Another friend suggested that she might have polycystic ovarian syndrome (PCOS).
Further history
- No significant medical history
- No family history of early menopause or genetic disorders
- Non-smoker
- Two to three standard drinks per week
- Taking a pregnancy vitamin supplement; no other medications or supplements
- Goes to gym for 45 minutes twice per week; no other regular exercise
- Up to date with cervical screening
- Up to date with immunisations including rubella, varicella, influenza and COVID-19
Physical examination
- Blood pressure 125/69 mmHg
- Height 172 cm, weight 71 kg, body mass index 24 kg/m2
- No clinical hyperandrogenism, thyroid or pituitary disease
- Urinary human chorionic gonadotropin (HCG) negative
- Susan’s GP arranges the following initial investigations:
- Serum HCG
- Follicle-stimulating hormone (FSH)
- Luteinising hormone (LH)
- Oestradiol
- Thyroid-stimulating hormone
- Prolactin
- Total testosterone, sex hormone binding globulin (SHBG)
- Pelvic ultrasonography
Investigation of secondary amenorrhoea
The most common causes of secondary amenorrhoea are pregnancy, hypogonadotropic hypogonadism, polycystic ovary syndrome, hyperprolactinaemia and thyroid dysfunction. Diagnostic criteria for POI relate to an elevated FSH level. If FSH and LH levels were low, hypogonadotrophic hypogonadism would be suspected. When POI is suspected, measuring an oestradiol level is a useful adjunct to confirm that it is low, supporting the diagnosis of POI. Measurement of total testosterone, which may be elevated in PCOS, is best performed using a sensitive method such as mass spectrometry. Further guidance on the investigation of secondary amenorrhoea can be found in the Therapeutic guidelines.1
Case continued
Susan returns for further evaluation after having pathology tests and pelvic ultrasonography performed.
Results are as follows (laboratory reference ranges vary between laboratories):
- HCG negative, FSH 51 IU/L (reference range follicular: 4–13 IU/L, mid-cycle: 5–22 IU/L, luteal: 2–8 IU/L, menopausal: 26–135 IU/L), LH 35 IU/L (reference range follicular: 2–10 IU/L, mid-cycle: 10–80 IU/L, luteal: 2–8 IU/L, menopausal: 8–59 IU/L), oestradiol <88 pmol/L (reference range follicular: <88–607 pmol/L, mid-cycle: 315–1828 pmol/L), luteal: 161–774 pmol/L, menopausal: <201 pmol/L), TSH normal, prolactin normal, testosterone low normal, SHBG normal
- Pelvic ultrasound – normal uterine size, ‘inactive ovaries’
Testing of FSH is repeated four weeks later; the result is 45 IU/L.
A diagnosis of POI is made. Arrangements are made for investigation into the cause and potential associated conditions, along with further assessment and support. Genetic and autoimmune tests are normal.
Susan’s main priority is fertility. She is referred to a reproductive endocrinologist for fertility assessment. Susan commences hormone replacement therapy (HRT) using transdermal oestradiol with cyclic oral micronised progesterone, being advised that this regimen is not contraceptive. After detailed investigation and counselling, Susan and her partner decide to explore the option of using a donor egg.
What is premature ovarian insufficiency?
POI is defined as the loss of ovarian function in women before the age of 40 years. Spontaneous POI affects approximately 4% of women globally with an increased prevalence in lower Human Development Index populations.2 However, specific Australian data are lacking. Causes of POI can be genetic, chromosomal, autoimmune, infectious or iatrogenic (Box 1). However, most cases of POI are idiopathic and are increasingly thought to be secondary to non-syndromic polygenic mutations that are not currently clinically detectable. Risk factors for POI include: presence of specific genetic variants, positive family history, autoimmune disease, earlier menarche, chemotherapy, radiotherapy, pelvic surgery, smoking and being underweight.3 Adverse early life experiences, including childhood abuse and parental divorce, and low socioeconomic status are associated with lower age at menopause, though specific data for a relationship with POI are lacking.3 Observational studies have shown that POI is associated with an increased risk of osteoporosis, cardiovascular disease, depression, anxiety, diabetes mellitus, cognitive dysfunction, dementia and increased mortality. There is emerging evidence implicating genetically associated accelerated ageing and oestrogen deficiency in these adverse health outcomes.4
| Box 1. Causes and associations of premature ovarian insufficiency (POI)32,33 |
Spontaneous POI
- Idiopathic – most common cause (>60% cases)
- Genetic (approximately 10–15% cases)
- X chromosome
- Turner syndrome (partial or complete loss of one sex chromosome)
- Fragile X premutation (FMR1 gene)
- Trisomy X
- Other: FOXL2, NR5A1, BMP15, FSHR
- Autoimmune (approximately 10–20% of cases)
- Addison’s disease
- Autoimmune thyroid disease
- Autoimmune polyglandular syndrome 1 and 2 (AIRE gene)
- Other: coeliac disease, type 1 diabetes mellitus, myasthenia gravis, connective tissue disorders, pernicious anemia, Crohn’s disease, primary biliary cirrhosis, vitiligo
- Metabolic
- Galactosaemia (GALT gene)
- 17α-hydroxylase deficiency
- Infectious
- Mumps oophoritis
- Human immunodeficiency virus, tuberculosis, cytomegalovirus, shigellosis, varicella
- Environmental
Iatrogenic POI*
- Chemotherapy: increased risk with cumulative dose, alkylating agents and combined chemotherapy plus radiotherapy (pelvic/whole body); includes the use of cyclophosphamide for non-cancer conditions such as systemic lupus erythematosus
- Radiotherapy: dependent on cumulative dose and field of exposure
- Ovarian surgery: bilateral oophorectomy
|
| *Frequency varies according to the population studied |
Diagnosis of premature ovarian insufficiency
A diagnosis of POI should be considered in any woman under 40 years presenting with oligo/amenorrhoea. Adolescents may present with primary amenorrhoea. Women may not necessarily experience menopausal symptoms such as hot flushes. The reasons for the lack of menopausal symptoms are unclear, but it may be due to intermittent ovarian function or lack of oestrogen priming, especially with primary amenorrhoea. Diagnostic criteria for POI are: FSH levels >25 IU/L on two occasions at least one month apart following 4–6 months of oligo/amenorrhoea, with exclusion of secondary causes of amenorrhoea (Figure 1).5,6 Anti-Müllerian hormone (AMH) levels are not currently recommended for the diagnosis of POI.6,7 The diagnosis is often delayed as women or their clinicians do not consider POI as a potential cause of oligo/amenorrhoea.6 ‘Post-pill amenorrhoea’ is sometimes blamed for a lack of normal menstrual cycles following cessation of the COCP. However, with the current formulations of COCPs, although conception rates may be lower in the first three months post cessation of the COCP, they have normalised by 12 months.8 Taking the COCP may mask an underlying menstrual disorder. Thus, women with four months of amenorrhoea post COCP cessation should be investigated for POI and not assumed to have post-pill amenorrhoea.
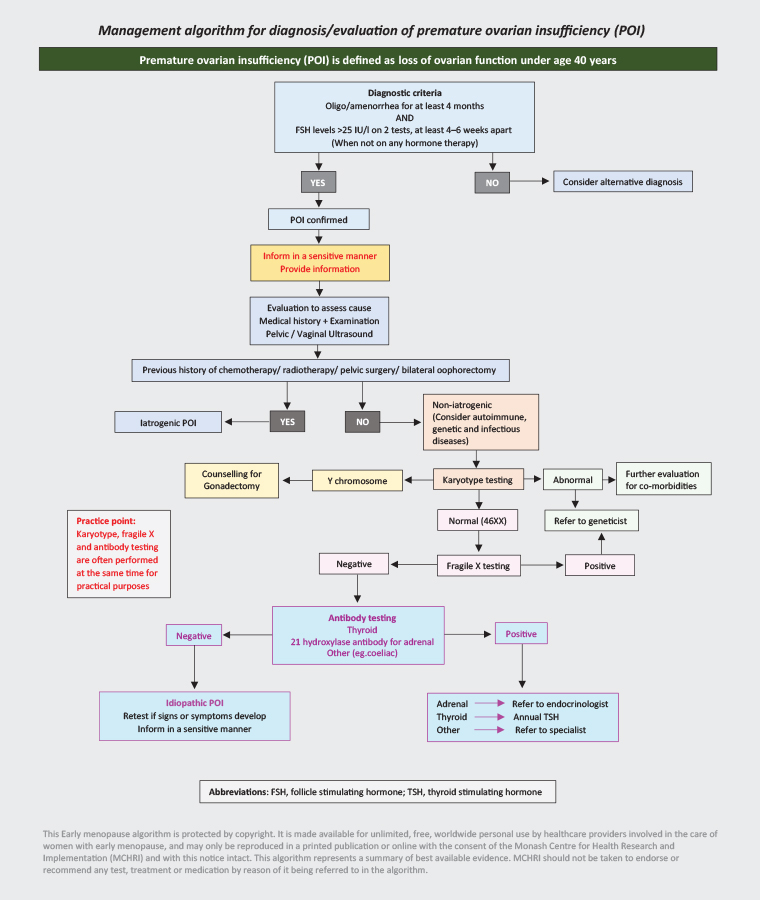
Figure 1. Premature ovarian insufficiency diagnosis evaluation algorithm. Click here to enlarge
Reproduced with permission from Monash Centre for Health Research and Implementation (MCHRI), Monash University, Clayton, Victoria, Australia
(www.monash.edu/medicine/sphpm/mchri/research/themes/womens-and-childrens-public-health/early-menopause-research). Copyright © MCHRI.
The diagnosis of POI can be unexpected and devastating for women and needs to be communicated in a sensitive manner. Psychological support should be offered. Referral to an appropriate endocrinology or gynaecology specialist is recommended. Once the diagnosis is made, a comprehensive assessment should be undertaken. This includes evaluation for a cause (Figure 1) and assessment of overall health, including bone health, cardiovascular disease prevention, fertility issues and psychological health (Figure 2).5,6 Where possible, a multidisciplinary team approach is beneficial.
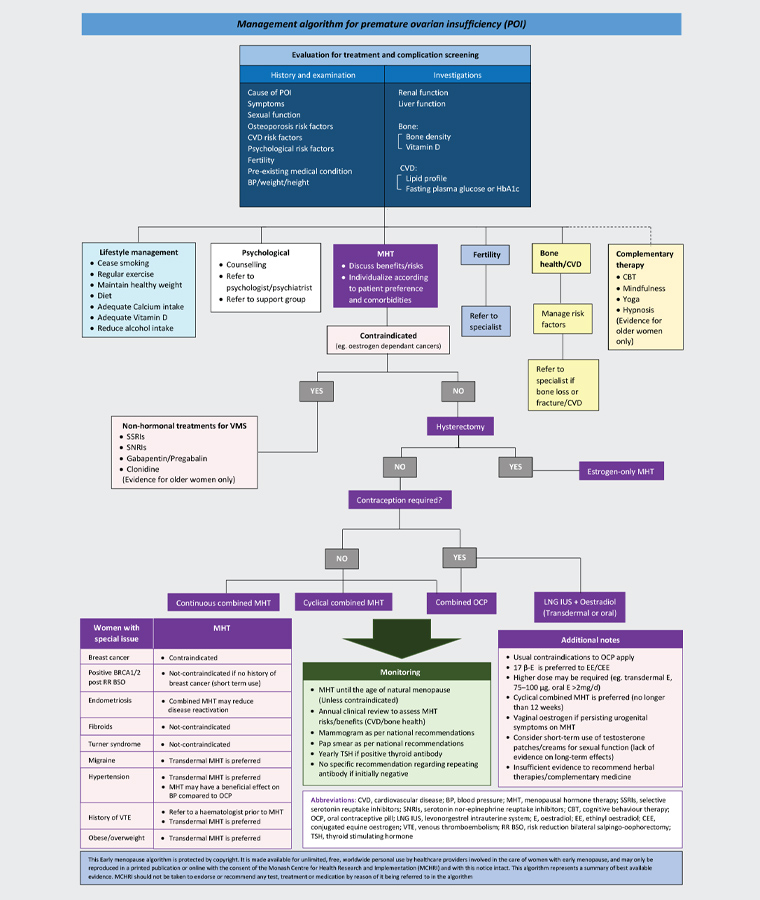
Figure 2. Premature ovarian insufficiency (POI) management algorithm. Click here to enlarge
Bone density testing should be repeated regularly. Although there is no agreed recommended interval, we suggest every second year. Referral to a lipid specialist should be considered with significant hypertriglyceridaemia (eg at increased risk of pancreatitis) or persisting with use of transdermal hormone therapy. The terms hormone replacement therapy (HRT) and menopausal hormone therapy (MHT) are often used interchangeably. In the case of POI, the term HRT is more appropriate.
Reproduced with permission from Monash Centre for Health Research and Implementation (MCHRI), Monash University, Clayton, Victoria, Australia
(www.monash.edu/medicine/sphpm/mchri/research/themes/womens-and-childrens-public-health/early-menopause-research). Copyright © MCHRI.
Management of premature ovarian insufficiency
Management of POI (summarised in Figure 2) is directed at:
- pubertal induction, if required
- relief of menopausal symptoms, if present
- chronic disease prevention, especially osteoporosis and cardiovascular disease
- psychological health
- fertility
- the cause of POI, if identified.
Hormone replacement therapy
Individualised HRT, unless contraindicated, should be instituted promptly and continued until at least the usual age of menopause6 to relieve symptoms and decrease the risk of adverse health effects. HRT is the preferred term (rather than menopausal hormone therapy [MHT]) in POI because the therapy is for a hormone deficiency. Although the optimal regimen is unknown, higher doses of oestrogen are usually required, especially for osteoporosis prevention.9,10 A recent study suggested that continuous use of the COCP increased spine and maintained femoral neck bone density; however, bone loss was observed with low-dose HRT, non-continuous COCP (that is taking inactive tablets) or no therapy.9,10 Data are lacking regarding the role of testosterone therapy in POI management.
Fertility management
Assessing low ovarian reserve
AMH is produced by ovarian granulosa cells and is a serum biomarker of ovarian reserve. It is useful in the assessment of subfertility and for planning of fertility management because of its intra- and inter-cycle stability.11–14 AMH levels fall with age in ovulatory women and become undetectable approximately five years prior to a rise in FSH and diagnosis of natural menopause.15,16 Low AMH can still occur in women with regular menstrual cycles and low ovarian reserve, as well as those with POI, and it therefore cannot be used to diagnose menopause.13
The antral follicle count (AFC) is the number of follicles measuring 2–10 mm in the early follicular phase seen on transvaginal ultrasonography.17 The AFC can be used to assess ovarian reserve as it correlates with the pool of follicles remaining in an ovary and with the AMH level.17
Predicting POI and fertility preservation
Currently there are no established methods of accurately predicting POI except in the instance of a known mutation, such as Fragile X premutation.6
Fragile X syndrome (FXS) is an X-linked dominant single-gene condition in which a person has an expanded set of CGG repeats in the FMR1 gene, located on the long arm of the X chromosome (Xq27.3). Having fewer than 45 CGG repeats is considered normal, 45–54 is intermediate, 55–200 is a premutation and >200 is a full mutation (FXS). In males, FXS is the most common cause of inherited intellectual disability. Those (mostly males) with a premutation are at risk of tremor ataxia syndrome; in females, 20% will develop POI and diminished ovarian reserve. Early signs of abnormal ovarian function in premutation carriers include irregular and shorter menstrual cycles.18–20 Carriers also have higher follicular and luteal FSH levels and a lower AMH level when compared with age-matched controls, even as early as 18 years of age.21
Women may present with menstrual cycle irregularities prior to typical menopausal symptoms.6 It is important to investigate for symptoms of oestrogen deficiency in women with oligomenorrhoea or amenorrhoea.6 In women with oligomenorrhoea or amenorrhoea, AMH levels have been found to be elevated in the subset with PCOS and low in those with POI, and therefore they may be useful in investigating irregular menstrual cycles.22 AMH is a more sensitive biomarker for predicting time to last menstrual cycle when compared with inhibin B or FSH; however, it remains imprecise in young women for predicting POI.7
Women at risk of developing POI should be referred for counselling (including pregnancy planning/timing) and consideration of fertility preservation. This includes women who are to receive gonadotoxic therapies, women with autoimmune disorders such as Addison’s disease or type 1 diabetes mellitus and women with known genetic mutations, such as FMR1 premutation or Turner syndrome. Administration of gonadotropin-releasing hormone analogues may help prevent chemotherapy-induced POI in women with early breast cancer23 or lupus nephritis.24 Oocyte and ovarian tissue cryopreservation are recognised fertility-preservation techniques.25,26 Instead of pursuing pregnancy, women may also elect to adopt or live childfree.
Options for achieving pregnancy
Spontaneous ovulation can occur after a diagnosis of POI, and approximately 5% of women will have a spontaneous pregnancy after a diagnosis of POI.27 For women who do not wish to rule out the option of spontaneous conception, it is appropriate to prescribe an HRT formulation that does not act as a contraceptive. For other women, contraception is recommended.6
In the case of diagnosed POI, often the opportunity for fertility preservation has already passed.6 For women with impending POI but a sufficient ovarian reserve, in vitro fertilisation (IVF) with their own oocytes may be possible.28 Often women in this situation require higher doses of gonadotropins for ovarian stimulation and have a poor response, with fewer oocytes able to be retrieved.28 They may require multiple cycles of IVF to conceive.28
For the majority of women with POI, and those with undetectable AMH levels, oocyte or embryo donor conception is required for pregnancy. Of note, using oocyte donation from the sister of a patient with POI unfortunately carries a higher risk of cycle cancellation (30.8%, compared with 6.6% when from an anonymous donor).29
Once pregnancy is achieved, POI in itself does not increase maternal or neonatal risks.6,30,31 However, the underlying cause of POI may have an impact on the pregnancy; for instance, the increased risk of obstetric complications in women who have had high doses of pelvic radiotherapy, or cardiac complications in women with Turner syndrome.6 These risks should be considered in pre-pregnancy counselling, and patients referred to appropriate high-risk clinics. Women with inheritable genetic conditions should also consider having genetic counselling prior to pregnancy.
Conclusion
POI – the loss of ovarian function in women under 40 years of age – may occur spontaneously or following medical therapy. POI has multiple adverse short- and long-term impacts on women’s psychological and physical health. Women may present with oligo/amenorrhoea or infertility. Diagnosis is determined by elevated FSH levels but is often delayed. Individualised HRT should be instituted promptly and continued until the usual age of menopause. Women at risk of POI should be referred for counselling and potential fertility preservation via oocyte or ovarian cryopreservation. For most women with POI, oocyte or embryo donor conception is required for pregnancy.