In Australia, the all-encompassing role of general practice in managing acute and chronic conditions, while also delivering preventive healthcare to promote and maintain optimal health, is well recognised as challenging but critical to maintaining the health of all Australians.1 The Royal Australian College of General Practitioners’ (RACGP) Red Book emphasises ‘that mid-life is particularly a time of determining patient risk factors and offering screening for health conditions’,1 whereas the RACGP’s Green Book recognises that evidence-based strategies are needed to overcome the challenges of implementation.2
In our opinion, there is much scope to use risk prediction methodology for chronic obstructive pulmonary disease (COPD), the leading cause of respiratory morbidity and mortality, given the use of risk prediction tools in the diagnostic and intervention pathways for cardiovascular disease and type 2 diabetes. Detecting COPD earlier has been highlighted in the recently released first Australian COPD blueprint, Transforming the agenda for COPD: A path towards prevention and lifelong lung health,3 and by the 2022 Lancet Commission.4 The latter proposed a new diagnostic algorithm and re-emphasised spirometry as the first-line test before chest imaging (Figure 11 in Stolz et al4). However, given that the use of spirometry was restricted during the COVID-19 pandemic, there is now an urgent need to re-establish timely access to spirometry by general practitioners (GPs) so that diagnoses such as COPD are confirmed promptly. Given the barriers of recommencing office/clinic spirometry in this living-with-COVID-19 era, this could be aided by triaging patients for pre- and post-bronchodilator spirometry through risk stratification.
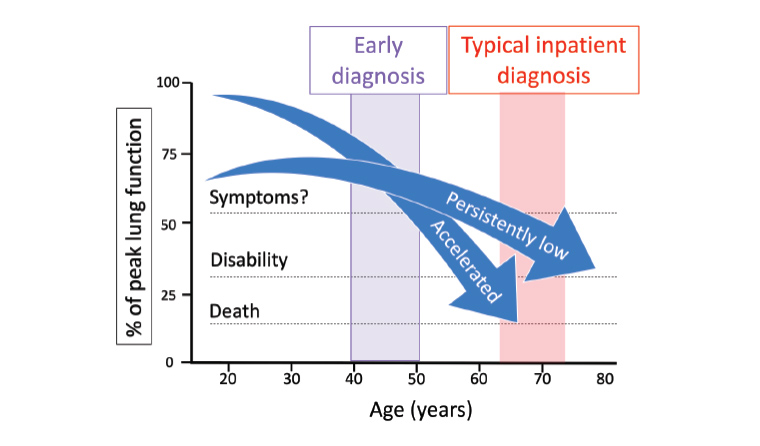
Figure 1. PRECURSOR’s aim to detect COPD earlier. The superimposed schematic representation of two disadvantaged lung function trajectories has been informed by the authors’ original work.6 Adapted from Fletcher and Peto, with permission from the BMJ Publishing Group.7
COPD, chronic obstructive pulmonary disease; PRECURSOR, Predicting Your Patient’s Risk of Airway Obstruction.
GPs will have many patients aged ≥60 years who first presented acutely to hospital with a severe exacerbation before a diagnosis of COPD was made.5 However, it is not commonly known that susceptible individuals on track to develop COPD from gradually declining lung function6 can be detected years earlier by spirometry (Figure 1). Furthermore, compared with diagnosed COPD, people with undiagnosed COPD are at a much-increased risk of acute exacerbations, pneumonia and premature death, even if they do not have symptoms.8 Many symptomatic patients with spirometric evidence of COPD remain undiagnosed in primary care settings.9 Thus, we believe the lack of a suitable COPD risk assessment tool and a clear pathway to objective testing contribute to diagnostic delays and deferment of best practice respiratory care. This includes smoking cessation strategies, vaccination, COPD action plans, maintaining healthy weight and promotion of physical activity, as well as indicated pharmacotherapies.
We propose a standardised risk assessment for COPD for ‘younger’ high-risk individuals that could be used in the 45- to 49-year-old health check or as a standalone tool for adults aged in their 40s. Current guidelines and position papers (eg RACGP,1 COPD-X,10 Lung Foundation Australia [LFA]11) recommend pre- and post-bronchodilator spirometry to be considered for adults with respiratory symptoms and/or pollutant exposures, such as smoking. However, undertaking such ‘opportunistic’ case finding is challenging in busy general practice. It would be more feasible to select smokers and people with asthma at high risk by using a risk calculator, and to target younger patients to trial potentially disease-modifying strategies earlier in the disease course.12 This viewpoint is shared by world experts (Figure 2 in Stolz et al4) and advocated by our peak lung health consumer body, LFA.3
We have developed a validated risk prediction model using questionnaire data from adults aged in their 40s to ‘predict COPD’ in their 50s called PRECURSOR (Predicting Your Patient’s Risk of Airway Obstruction),13 with no need for an examination or blood tests. For example, a 43-year-old unskilled worker who smoked 20 cigarettes/day since early adolescence and recently wheezed has a predicted probability of 29% for clinical COPD in their 50s if they continue smoking. However, the ‘risk’ odds would be close to fivefold lower for similar smokers who had quit one year earlier (or a 7.4% probability of having COPD in their 50s). These risk predictions could inform patient selection for spirometry referral to confirm a diagnosis of COPD. Furthermore, providing different smoking scenarios of quitters compared with continuing smokers may be a critical motivating factor for smokers to quit. Obtaining an individualised risk prediction for any combination of history of smoking, asthma, job and symptom elicited by the risk tool is possible, and examples of other high-risk scenarios have been presented in the paper’s online supplement.13
The performance of this COPD risk model13 is comparable to that of other questionnaires without peak expiratory flow or microspirometer measurements (eg the COPD Diagnostic Questionnaire,14,15 the COPD Population Screener14,16 and the COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk tool;17 see Table 1). However, an advantage of PRECURSOR is that it has been developed and validated using data from two of the largest general population respiratory cohorts worldwide and designed for younger people, aged in their 40s, who may be relatively asymptomatic.
| Table 1. Comparisons between the PRECURSOR toolA and other chronic obstructive pulmonary disease questionnaires |
| Features |
CDQ15 |
COPD-PS16 |
CAPTURE tool17 |
PRECURSOR toolA,13 |
| Derivation population |
Smokers of general populations |
General practice and specialist sites |
General population aged ≥40 years |
General population of adults aged in their 40s |
| Internal validation (AUCROC [95% CI]) |
81.6% (n/a) |
81.0% (n/a) |
79.5% (n/a) |
80.8% (80.0, 81.6) |
| External validation for questionnaire only (AUCROC [95%CI]) |
72% (64, 78)14 |
77% (63, 85)14 |
n/a |
75.6% (75.4, 75.8)13 |
| External validation with peak flow measurement (AUCROC [95%CI]) |
n/a |
n/a |
0.81% (0.77, 0.85) |
n/a |
| Designed to predict ‘early’ COPD in ‘young’ people aged 40–49 years, who may be asymptomatic |
No |
No |
No |
Yes |
| Based on longitudinal data (like other risk‑calculatorsB) |
No |
No |
No |
Yes |
| Projected 10-year risks for continued smoking vs quitting |
No |
No |
No |
Yes |
| Calculates pack-year smoking history |
No |
No |
No |
Yes |
| Digital tool can be completed by phone interview, with a printable PDF report |
No |
No |
No |
Yes |
| Potential for shared decision making to undergo spirometry |
Yes |
Yes |
Yes |
Yes |
AUndergoing productionisation.
BRisk prediction calculators for cardiovascular disease and diabetes.
AUCROC, area under the receiver operating characteristic curve; CAPTURE, COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COPD-PS, COPD Population Screener; CDQ, COPD Diagnostic Questionnaire; LMIC, low- and middle-income countries; n/a, not available; PRECURSOR, Predicting Your Patient’s Risk of Airway Obstruction. |
The potential for the implementation of PRECURSOR into Australian clinical practice is currently being evaluated. Furthermore, this COPD-risk model is currently undergoing translation as a web-based application accessible to patients aged in their 40s who, for example, could be invited via SMS, email or letter to complete the risk tool online, with a report generated and returned to the practice for review. This systematic approach to actively finding ‘young’ middle-aged patients with COPD holds promise to create preventive opportunities and best practice respiratory care in a timely manner. Please watch this space.